Determine Whether Each Compound Is Soluble or Insoluble in Water.
KBr AgBr BaSO4 Al2SO43 AlOH3. The solubility rules shows which compounds are soluble in water.
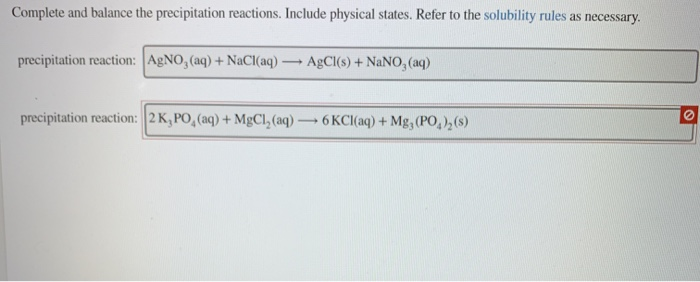
Solved Predict Whether The Compounds Are Soluble Or Chegg Com
Except for a few exceptions Inc is not one of them was included is soluble and all sulfides are insoluble and there are a few exceptions.
. The Top Row Shows The Cation In. Predict whether the following compounds are soluble or insoluble in water KNO3 CoBr2 AgNO3 AgBr BaSO4 NiCO3. Consult the solubility table to answer the question Soluble in water.
Determine whether each compound is soluble or insoluble in water. In bespeak to identify if one ionic compound is dissolve or not us can check the solubility rules. All nitrates are soluble.
Therefore AgI is insoluble. Ions are listed as an exception. Theres a scalability rule that states all potassium or alkali metal salts are soluble and one that states all nitrates are soluble so this compound would be soluble.
So this would be insoluble. Consult the solubility table to answer the question. Determine whether each compound is soluble or insoluble in water.
Determine the percent yield. Classify each of the possible reactions according to whether a precipitate will form or will not form. The Results Are Shown In The Chart Below.
These are for common cations too long for me to type out. For each soluble compound identify the ions present in solution. KBr Al2SO43 Insoluble in water.
Soluble Insoluble Answer Bank Answer Bank LOH Al OH AgBr Hy CI Сасо NaF. Therefore a solution that conducts electricity well. -m BaSO4 315g.
KBr KNO3 Insoluble in water Ni3 PO42Al OH3AgBr Solubility Rules. Consult the solubility table to answer the question. According to the solubility rules all chlorides and bromides are soluble in water except those of silver and mercuryThis explains why most of the chlorides and bromides are designated as soluble.
Up to 24 cash back 757 Determine whether each compound is soluble or insoluble. Determine whether each compound is soluble or insoluble in water. Find step-by-step Chemistry solutions and your answer to the following textbook question.
If It Does Dissolve No Mark Is Made. One of my favorite chemistry books General Chemistry by Linus Pauling has some very nice rules of thumb for predicting solubility on page 453 Dover edition. Express your answer as a chemical formula.
A solution containing a solute that dissociates into ions. For the dissolve compounds compose the ions developed when the salt disappear in water. Then we have led to chloride.
Determine the limiting reactant. Chemistry questions and answers. All nitrates are also soluble in waterThe hydroxide of copper is not soluble in water as well as the.
Consult the solubility table to answer the question Soluble in water Insoluble in water KCI BSO AL SO MOOH Answer Bank Incorrect. Predict whether each of the following compounds is soluble in water. Determine whether each of the following compounds is soluble or insoluble.
Mostly soluble no exceptions. 100 5 ratings Soluble in water. Determine whether each compound is soluble or insoluble in waterA.
The answer is that it is soluble in water. Soluble in water Insoluble in water. A NaC 2 H 3 O 2 soluble NaC 2 H 3 O 2 aq Na aq C 2 H 3 O-aq b SnNO 3 2 soluble SnNO 3 2 aq Sn 2aq 2 NO 3-aq c AgI insoluble AgIs d Na 3 PO 4 soluble Na 3 PO 4 aq 3 Na aq PO 4 3-aq.
Not soluble in water. A set of empirical rules used to determine whether an ionic compound is soluble. An X Indicates That The Compound Does Not Dissolve In Water.
Determine whether each compound is soluble or insoluble in water. Is K2SO4 Potassium sulfate soluble or insoluble in water. Each of the following compounds is soluble in water.
The following are the solubility rules for common ionic solids. Determine the theoretical yield. Answer 1 of 4.
If there two rules appear to contradict each other the preceding rule takes precedence. Pretty much all hydroxide except for those forms with alkali metals are insoluble so iron three hydroxide is insoluble while chlorides are soluble. Soluble in water Insoluble in water Answer Bank KCI Hg Cl AgNO3 ZnPO42 Nico.
This is the last question that I am having a hard time with In a. Determine whether each compound is soluble or insoluble in water. .
Determine whether each compound is soluble or insoluble in water. We space asked todetermine whether every of the offered compounds is soluble or insoluble. It is an ionic compound which readily dissociates into.
Determine the limiting reactant the theoretical yield and the percent yield. Theres a rule that states all chloride salts are soluble but lead to is an exception. The first compound is potassium nitrate.
According to solubility rules all iodide anions are soluble but Ag. Several Solid Compounds Are Placed In Water To Determine If They Are Soluble.
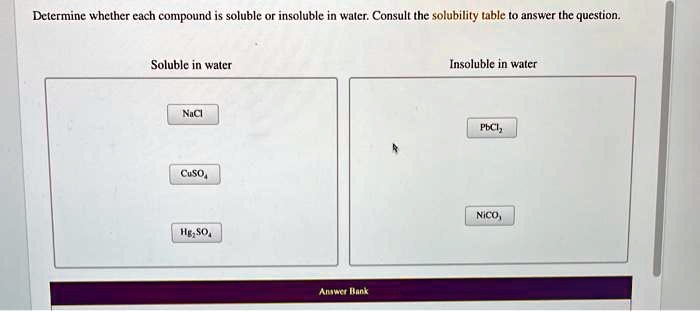
Solved Determine Whether Cach Compound Is Soluble Isol Uble In Waler Consult The Solubility Table To Answer The Question Soluble In Water Insoluble In Water Ncl Pici Cso Nico Anact Want
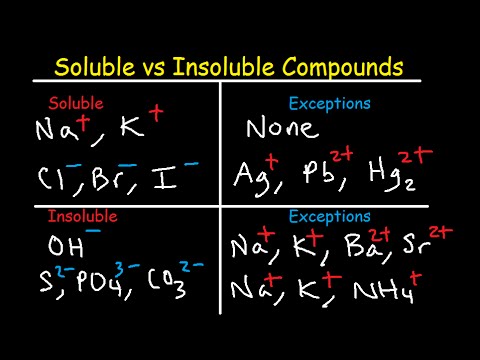
Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube

Solubility Rules Chemistry Video Clutch Prep

Chemistry The Central Science Chapter 4 Section 2

How To Determine Solubility 14 Steps With Pictures Wikihow

Correctly Classify Each Of The Following Compound As Highly Soluble Or Insoluble In Water 1 Nacl 2 Brainly Com

Solved Determine Whether Each Compound Is Soluble Or Chegg Com

Solved Determine Whether Each Compound Is Soluble Or Chegg Com

Solubility Introduction To Chemistry

Solved Determine If Each Compound Shown Is Soluble Or Chegg Com

Solved Determine Whether Each Compound Is Soluble Or Chegg Com

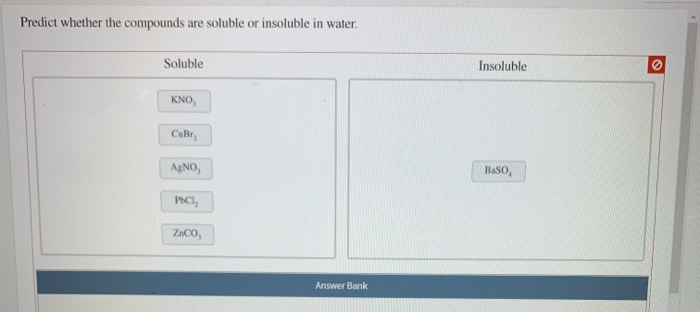
Solved Predict Whether The Compounds Are Soluble Or Chegg Com

Determine If Compounds Are Soluble In Hexane Youtube

Chemistry The Central Science Chapter 17 Section 5

Solved Determine Whether Each Compound Is Soluble Or Chegg Com



Comments
Post a Comment